New Products
18 panel drug screen with ADLTX
As Low As $2.99
| 18 Panel Drug Tested |
| AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300, OXY/100, BUP/10, MDMA/500, THC/50, BAR/300, PCP/25, TRA/100, KET/1000, FEN/20, ETG/500, KRAT/500 & 3 adulterations ADLTX (Specific Gravity, pH, and Creatinine) |
14 Panel Drug Test with hCG Rapid Urine Drug Test
As Low As $2.29
| Drugs Tested |
| ALC, AMP, BAR, BUP, BZO, COC, FYL, MDMA, MET, MTD, OPI, OXY, THC & hCG |
13 panel drug screening FYL, EtG & K2
As Low As $2.29
| Drugs Tested |
| AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300, OXY/100, BUP/10, THC/50, FEN/20, PCP/25, ETG/300, K2/50 |
22 panel drug test
As Low As $3.89
| 22 Panel Drug Tested |
| AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300, OXY/100, BUP/10, MDMA/500, THC/50, BAR/300, PCP/25, TRA/100, KET/1000, K2/50, FEN/20, ETG/300, KRA/500, DELTA8/25, XYL/1000, ZAZA/500 & 3 adulterations ADLTX (Specific Gravity, pH, and Creatinine) |
Boson covid test
As Low As $1.69 per test
Expiration Date August 2024
Authorized by FDA under an Emergency Use Authorization (EUA)
SHIPPING SAME DAY
| Test info | |
| Covid test | |
| Nucleocapsid Protein Antigen | |
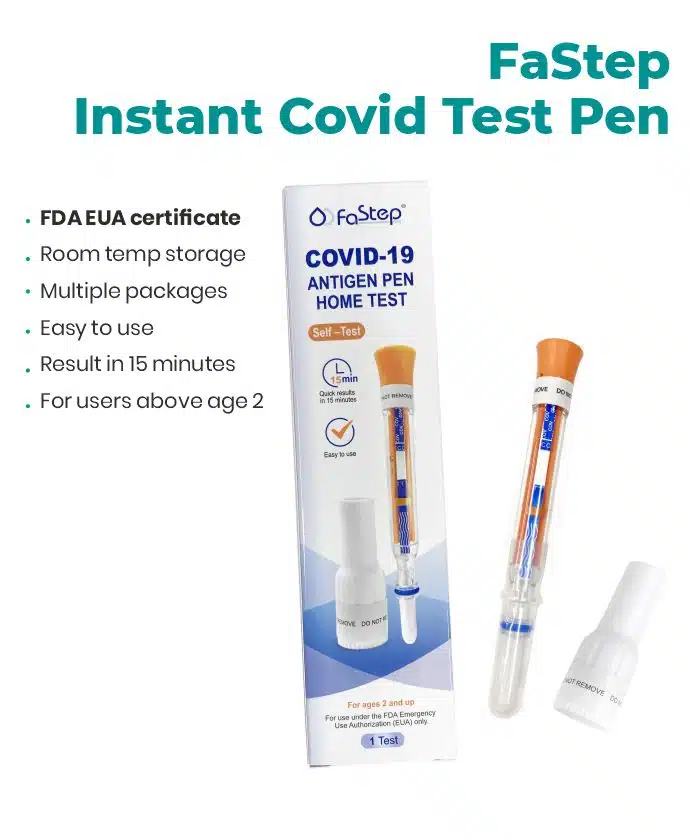
Covid Test – FaStep instant covid test Antigen Home Test
As Low As $1.79 per test
Authorized by FDA under an Emergency Use Authorization (EUA)
SHIPPING SAME DAY
| Test info | |
| Covid test | |
| Covid-19 Antigen Pen | |
12 panel drug test cup TCA
As Low As $1.89
BLOWOUT SALE
| 12 Panel Drug Tested |
| AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300,OXY/100, BUP/10, MDMA/500, THC/50, BAR/300, TCA/1000 |
14 Panel Drug Test Cup with EtG & TRA – Rapid Urine Drug Test
As Low As $2.29
| 14 panel drug test list |
| AMP, BAR, BUP, BZO, COC, MAMP (MET), MDMA, MTD, OPI300 (MOP), OXY, THC, TRA, FYL & ETG |
10 Panel UA Drug Test With FYL (Fentanyl)
As Low As $1.59
| Drug Tested |
| AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300, OXY/100, BUP/10, THC/50, FYL/20 |
12 panel drug test cup TCA
As Low As $1.89
BLOWOUT SALE
| 12 Panel Drug Tested |
| AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300,OXY/100, BUP/10, MDMA/500, THC/50, BAR/300, TCA/1000 |
12 panel cups with PCP
As Low As $1.89
| Drugs Tested |
| AMP, BAR, BUP, BZO, COC, MAMP (MET), MDMA, MTD, OPI300 (MOP), OXY, THC & PCP |
Xyl Test Strip – Xyl (XYL) Drug Test Strip
As Low As $0.69
| Cutoff level | |
| Xylazine | 1000 ng/ml |
ZAZA drug test – Tianeptine drug test
As Low As $0.99
| Cutoff level | |
| Tianeptine | 500 ng/ml |
16 panel drug test cup with KRA, EtG and FYL
As Low As $2.79
| Drugs Tested |
| AMP, BAR, BUP, BZO, COC, MAMP (MET), MDMA, MTD, OPI300 (MOP), OXY, THC, TCA, PCP, EtG, KRA, FYL (Fentanyl) |
Drug Testing Cup
BLOWOUT SALE
All Drug cup Test you need are in one place.
Most affordable supplier of drug test kits on the market.
When it comes to drug testing solutions, efficiency, accuracy, and cost-effectiveness are paramount. Look no further — our comprehensive range of Drug Testing Cups is tailored to meet your bulk testing needs. From wholesale pricing to the best deals in the business, we’re your trusted partner in maintaining a safe and drug-free environment.
Bulk Drug Testing Cups: A Wise Investment
Unbeatable Wholesale & Bulk Pricing:
Our commitment to affordability sets us apart. We understand that cost considerations play a crucial role in your decision-making process. That’s why we offer wholesale and bulk pricing options designed to fit your budget without compromising on the quality of our products. When you choose us, you’re choosing the best price in the business.
Tailored Solutions for Every Need:
Whether you require urine drug test cup for workplace screenings, healthcare facilities, or educational institutions, our diverse range has you covered. Our bulk offerings extend to various panel options, ensuring you have the flexibility to choose the right cup for your specific requirements.
Best Selling 12-Panel Cups:
Experience the epitome of comprehensive drug testing with our best-selling 12-panel cups. These cups are industry favorites instant drug test, providing simultaneous detection of a wide range of substances, including but not limited to THC, Cocaine, Opiates, Methamphetamines, and more. Enhance your screening capabilities with precision and ease, all in a single, efficient test.
Why Choose Our Drug Testing Cups:
1. Uncompromised Quality:
Our drug testing cups are meticulously crafted, adhering to the highest industry standards. Each cup ensures accurate results, enabling you to make informed decisions swiftly.
2. User-Friendly Design:
Simplify your drug testing process with our user-friendly cups. Easy to handle and interpret, these cups are designed to streamline the testing experience for both professionals and non-professionals alike.
3. Confidentiality and Compliance:
We understand the importance of maintaining confidentiality and complying with industry regulations. Our drug testing cups are crafted with privacy and compliance in mind, ensuring that your testing process aligns with the necessary standards.
Order Now for a Seamless Bulk Buying Experience:
Ready to streamline your drug testing procedures with unbeatable deals? Trust our commitment to quality, affordability, and customer satisfaction. Ordering your drug testing cups in bulk has never been this seamless.
Don’t miss out on the best pricing in the business. Contact us today to discuss your bulk requirements and unlock a world of efficiency and cost savings with our top-of-the-line Drug Testing Cups. Your path to reliable, bulk drug testing solutions starts here.
12 Panel Drug Test Cup
with TCA
AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300, OXY/100, BUP/10, MDMA/500, THC/50, BAR/300, PCP/25
CLIA Waived
as low as $1.89 per cup
13 Panel Drug Test Cup
with FYL
AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300, OXY/100, BUP/10, MDMA/500, THC/50, BAR/300, TCA/1000, FYL/20
as low as $1.69 per cup
14 Panel Drug Test Cup
with FYL & EtG
AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300, OXY/100, BUP/10, MDMA/500, THC/50, BAR/300, TRA/200, FYL/20, ETG/500
as low as $2.29 per cup
13 Panel Drug Test Cup
with FYL & EtG
AMP/1000, OPI/300, MET/1000, BZO/300, COC/300, MTD/300, OXY/100, BUP/10, MDMA/500, THC/50, BAR/300, TCA/1000, FYL/FEN/20
one price $1.79 per cup
High-complexity testing of Illicit drugs including Xyl, Fen, Zaza
300+ in-network insurance
Lab Reps Wanted
We offer Special Pricing on Drug Testing Cups for Bulk Orders, you can even Create a custom cup specific to Your Company’s Needs!
Urine Drug Screen Cups
Drug Test Wholesale – Drug Test Cup
At DrugTestingCup, an American drug test company, we believe that fighting addiction doesn’t need to be expensive, that is why we offer affordable drug test kits that can be brought for less than $1. In addition to all, we ship your orders the same day and for a drug test in bulk orders every shipping is free.
We ensure that you never have to deal with stock issues when it comes to drug test cups. Our drug testing supplies have over 600,000 drug test kits for all drugs stocks ready.
Hence, when you book an order, whether it is 10 drug test cups or 100, we deliver all within just a few days. With a shelf life of 24 mounts, you can be sure that you get cheap drug test kits and quality products every time you order.
Why customer trust us
Latest Articles
Unraveling the Mystery of Tianeptine and Zaza: A Comprehensive Guide on Drug Testing and Usage
Unraveling the Current State of COVID-19: A 2023 Overview
Unveiling Certainly Most Commonly Used Drug Test Cup: The 12 Panel Cup
SAMHSA cutoff levels
FAQ
What do you need to know
Who we are?
We pride ourselves on our excellent customer service and our commitment to providing customers with quality products at bulk prices.
DRUG TESTING CUP is a family-owned and operated business based in West Palm Beach, Florida.
FDA approved, CLIA waived, easy to read, and affordable. One year later, and we are the fastest-growing drug testing supplier in Florida with over 500 accounts of commercial users.
Our 12 and 6-panel drug test cups are the lowest in the nation and we constantly receive feedback from our customers stating how happy they are with their savings. Some were paying as high as $5 per drug test cup, compared to $1.59 per drug test cup or lower for the same quality product when you buy wholesale drug tests.
We keep massive stock to ensure same-day shipping on orders placed before 4 pm EST, as well as cater to the needs of our bulk buyers, distributors and wholesalers.
We value the virtue of being good people and always take care to show respect and appreciation for our clients.
Our company takes pride in the highest level of quality service, and we work hard to serve our customers, large or small, with all their needs.
We cater to hundreds of clients nationwide, and we are growing every day.
While we’ve made a name for ourselves through our drug test cups, we are looking forward to supplying even more medical products at amazing prices.
Individually, each member of our staff has given back to the community in their own way, whether that be through donating thousands of dollars to charities or hosting fundraisers for terminally ill children.
We’re still a young company, but we’re looking forward to supporting our communities in bigger and more impactful ways as we grow.
Why buy Drug testing kit on wholesale
Where can I buy a drug test kits for sale?
The drug testing cup has a round design with a no-drip screw top lid, a peel-and-read label, and is leak-resistant. Each cup also contains a built-in temperature strip that is used to authenticate the donor’s urine.
The cup is extremely sensitive and conforms to SAMHSA cutoff levels. New lower cut-offs make this the ideal urine analysis test for pain management and addiction screening.
Each drug testing cup possesses a 99% accuracy rate and is also very comprehensive to use for at-home drug testing – it’s a fully integrated and self-contained screening cup.
What are cutoff levels
You can view a chart listing the cutoff levels for each of the tested drugs in the table below we set a list of our commonly used strips.
| Drug Name | Abbreviation | Cut-Off Level |
| Alcohol | ALC | 0.02% |
| Amphetamines | AMP | 1000 ng/mL |
| Barbiturates | BAR | 300 ng/mL |
| Benzodiazepines | BZO | 300 ng/mL |
| Buprenorphine | BUP | 10 ng/mL |
| Cocaine | COC | 300 ng/mL |
| Ecstasy | MDMA | 500 ng/mL |
| Fentanyl | FEN | 200 ng/mL |
| Marijuana | THC | 50 ng/mL |
| Methadone | MTD | 300 ng/mL |
| Methamphetamine | mAMP (or MET) | 1000 ng/mL |
| Morphine | MOR | 300 ng/mL |
| Opiates | OPI | 300 ng/mL |
| Oxycodone | OXY | 100 ng/mL |
| Phencyclidine | PCP | 25 ng/mL |
| Propoxyphene | PPX | 50 ng/mL |
| Synthetic Marijuana K2/Spice | K2/Spice | 5 ng/mL |
| Tramadol | TRA | 100 ng/mL |
| Tricyclic Antidepressants | TCA | 1000 ng/mL |
How does a drug test cup work?
Interpretation of results:
- POSITIVE: Only one colored band appears, in the control region (C). No colored band appears in the
test region (T) for the drug in question. A positive result indicates that the drug concentration exceeds
the detectable level. - NEGATIVE: Two colored bands appear on the membrane. One band appears in the control region (C)
and another band appears in the test region (T) for the drug in question. A negative result indicates that
the drug concentration is below the detectable level. - INVALID: Control band fails to appear. Results from any test which has not produced a control band at
the specified read time must be discarded. Please review the procedure and repeat it with a new test. If the
problem persists, discontinue using the kit immediately and contact your local distributor.
Who is using our drug testing cups
There is a variety of users that benefit from our drug test in bulk prices. When you buy drug tests in bulk from us you can expect prices to go down as far as 48%.
Rehabs
To make sure their patients are taking their treatment programs seriously and staying away from illegal substances, rehabs and drug treatment facilities require their patients to take drug tests.
Employment
To ensure health, safety, and productivity in the workplace, most employers typically require regular and random drug testing. They also require applicants to take a drug test as one of the prerequisites for hiring.
Athletic requirements
To ensure fair competition in all their athletic events, sports organizations, be they collegiate or professional, require athletes to take a drug test to rule out doping.
Legal purposes
Courts require people charged with DUI and other criminal activities to undergo drug tests.
Can I cheat drug test?
Drug test cups are designed with few security features. So cheating is really difficult.
There are cups with Adulterants that will show the presence of any chemical in the urine.
Also, there is a temperature strip that will notify about the drug test cup temperature or the low temperature of a sample.
And in the end, they are also transparent so the technician can see if you temper the sample.
How accurate is the drug test cups?
Our drug test cups are 99% accurate. Our drug testing cups and strips are reliable and sensitive to traces of drugs within the urine.
When a drug is detected, it will show up as a preliminary positive result on the drug testing device used. All positive results should be sent to a laboratory for verification.
How long does a drug test take?
Are your drug testing cups CLIA Waived and FDA approved?
Yes. Our drug testing cups are CLIA Waived and FDA approved.
How accurate are your drug test cups?
Our drug test cups are 99% accurate.
How do I store my drug testing cup?
- The drug testing cup should be stored at 60 and no more than 75 Fahrenheit until the expiry date printed on the sealed pouch.
- The drug test cup must remain in the sealed pouch until use.
- Do not freeze.
- Testing cups should be kept out of direct sunlight.
- Care should be taken to protect the components of the cup from contamination.
- Do not use if there is evidence of contamination or precipitation.
- Biological contamination of the dispensing equipment, containers, or reagents can lead to false results.
Online support.
We prefer to keep our support online for a few reasons:
- We can instantly view your account activity and details, and other information so we can troubleshoot effectively.
- It’s faster. We can quickly get to the bottom of your questions without putting you on hold.
- Online support keeps detailed records in one place. This ensures nothing gets lost in translation if we need to escalate your issue.
- We’ve received feedback that live chat support is similar to talking on the phone, as it involves real-time conversation.