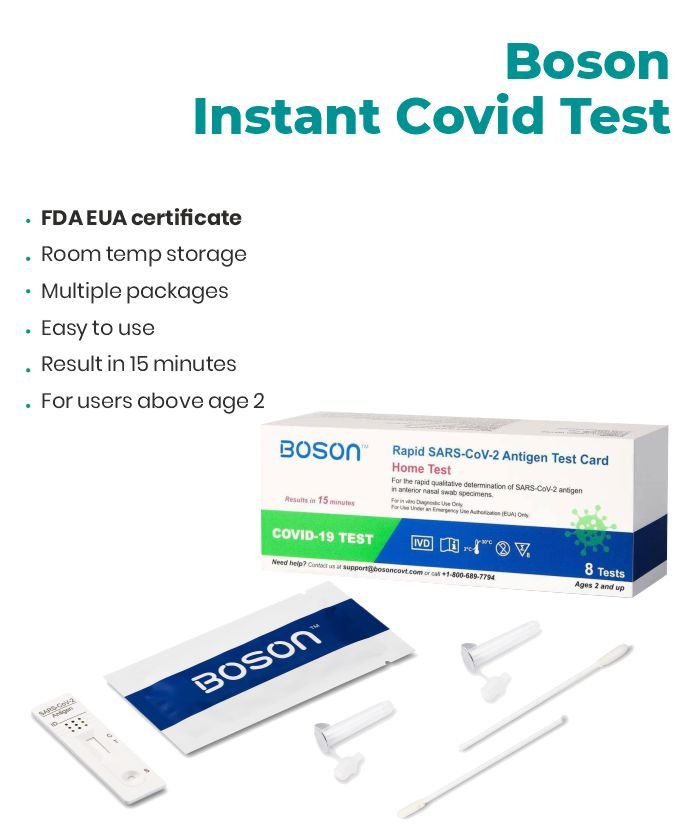
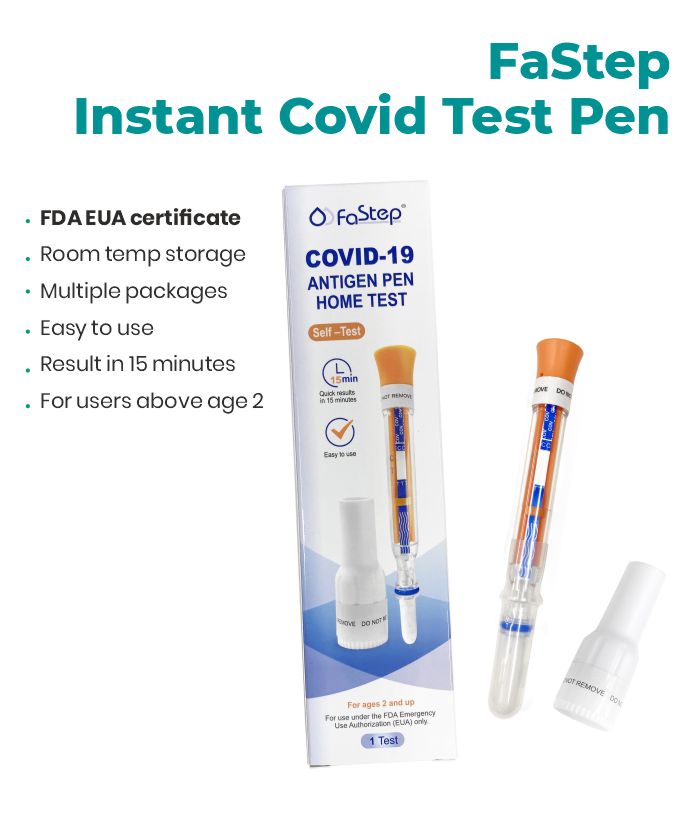
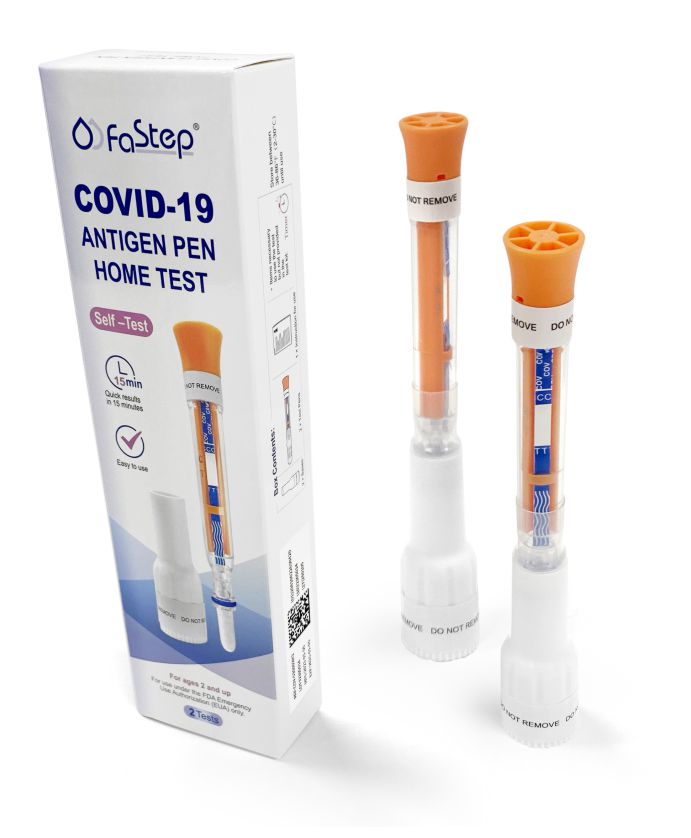
Accurate, fast, and easy to use. Quick Results, Lowest Prices.
Accurate, fast, and easy to use. Quick Results, Lowest Prices.
Accurate, fast, and easy to use. Quick Results, Lowest Prices.
High accuracy rate, 25 in a pack
Showing all 2 results
| Test info | |
| Covid test | |
| Nucleocapsid Protein Antigen | |
| Test info | |
| Covid test | |
| Covid-19 Antigen Pen | |
From Monday through Friday 9am to 5pm or by appointment.
You can order 24/7